Image
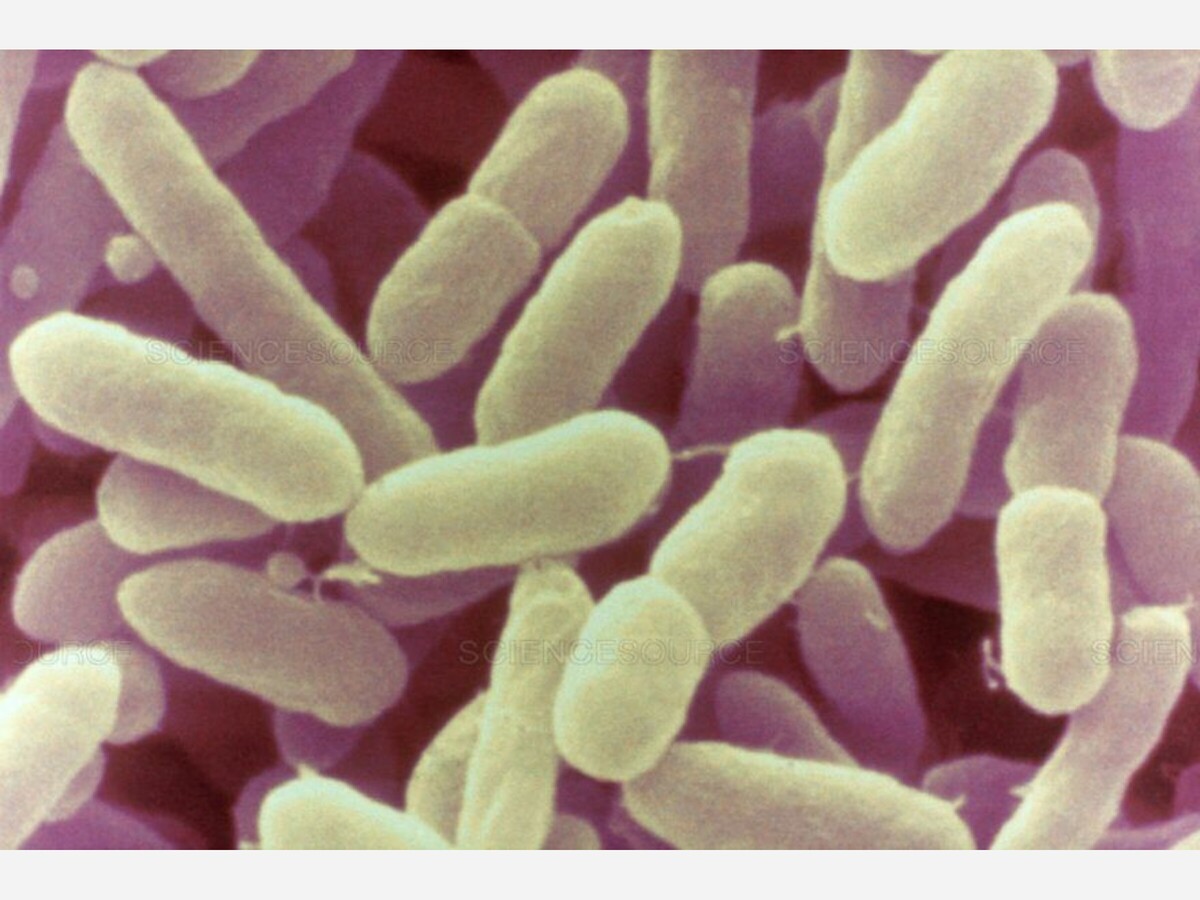

On November 15, 2022, the FDA announced and released an outline of a prevention strategy under development to prevent Cronobacter sakazakii illnesses associated with the consumption of powdered baby formula.
This prevention plan is being developed following a large-scale recall of powdered infant formula in the early part of 2022. Powdered infant formula was recalled because FDA investigators discovered unsanitary conditions in a manufacturing facility, which can contribute to Cronobacter sakazakii illnesses diagnosed in four babies.
The outline released today is intended to guide discussions during additional development of the FDA’s strategy to prevent Cronobacter sakazakii illnesses associated with the consumption of powdered baby formula.
In the forthcoming weeks, teams from across the FDA will be meeting with stakeholders to further confer, listen to their ideas, and finalize the prevention strategy.
Following this meeting, the FDA will release an updated outline of the powdered infant formula strategy on FDA.gov.
Cronobacter illness In infants, Cronobacter can cause severe meningitis or an infection in the bloodstream, medically known as sepsis, an infection of the membranes that protect and surround the brain. Meningitis caused by Cronobacter occurs exclusively among babies two months old.
This report was updated by Anita johnson-Brown
photo: Food Poison Journal