Image
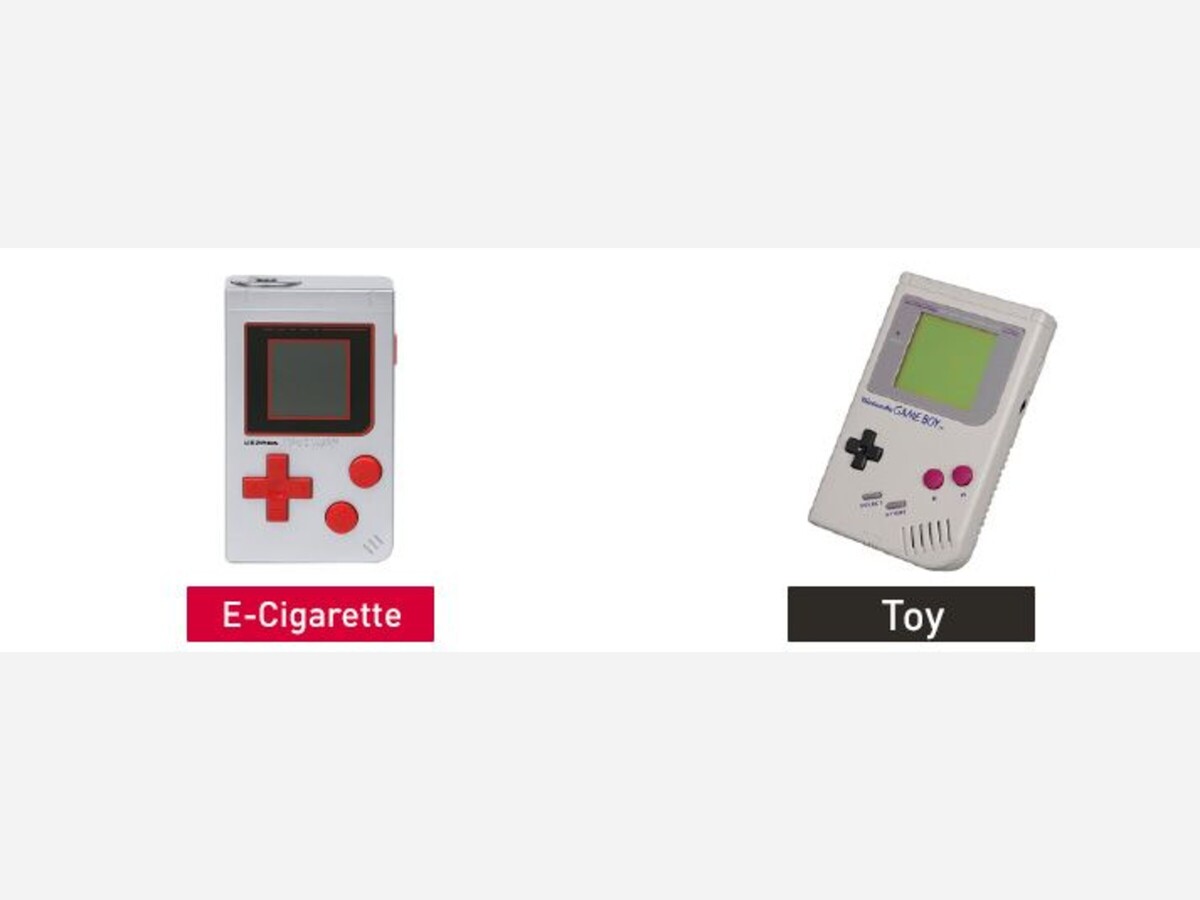
On November 16, 2022, the U.S. Food and Drug Administration issued warning letters to five firms for the unauthorized marketing of 15 different e-cigarette products. Each e-cigarette product is packaged to look like toys, food, or cartoon characters and is likely to promote use by youth.
None of the manufacturers submitted a premarket application for any of the unauthorized products.
The unauthorized products described in the warning letters include e-cigarettes that:
"The designs of these products are an utterly flagrant attempt to target kids,” said Brian King, Ph.D., M.P.H., director of the FDA’s Center for Tobacco Products. “It’s a hard sell to suggest that adults using e-cigarettes with the goal of quitting smoking need a cartoon character emblazoned across the front of the product in order to do so successfully.”
The FDA issued warning letters to:
The warning letters notify the recipients that e-cigarettes without a marketing authorization order are adulterated and misbranded and that selling or distributing these products to consumers in the U.S. is prohibited under the Federal Food, Drug, and Cosmetic (FD&C) Act. Failure to promptly correct the violations can result in additional FDA actions such as an injunction, seizure, and/or civil money penalties. In addition, products that appear to be misbranded or adulterated that are offered for import into the U.S. are at risk of being detained or refused admission. Retailers and distributors should communicate with their suppliers to discuss possible options for unauthorized products in their inventory.

This news report was shared by the U.S. Food and Drug Administration and has been updated by Anita Johnson-Brown, Editor