Image
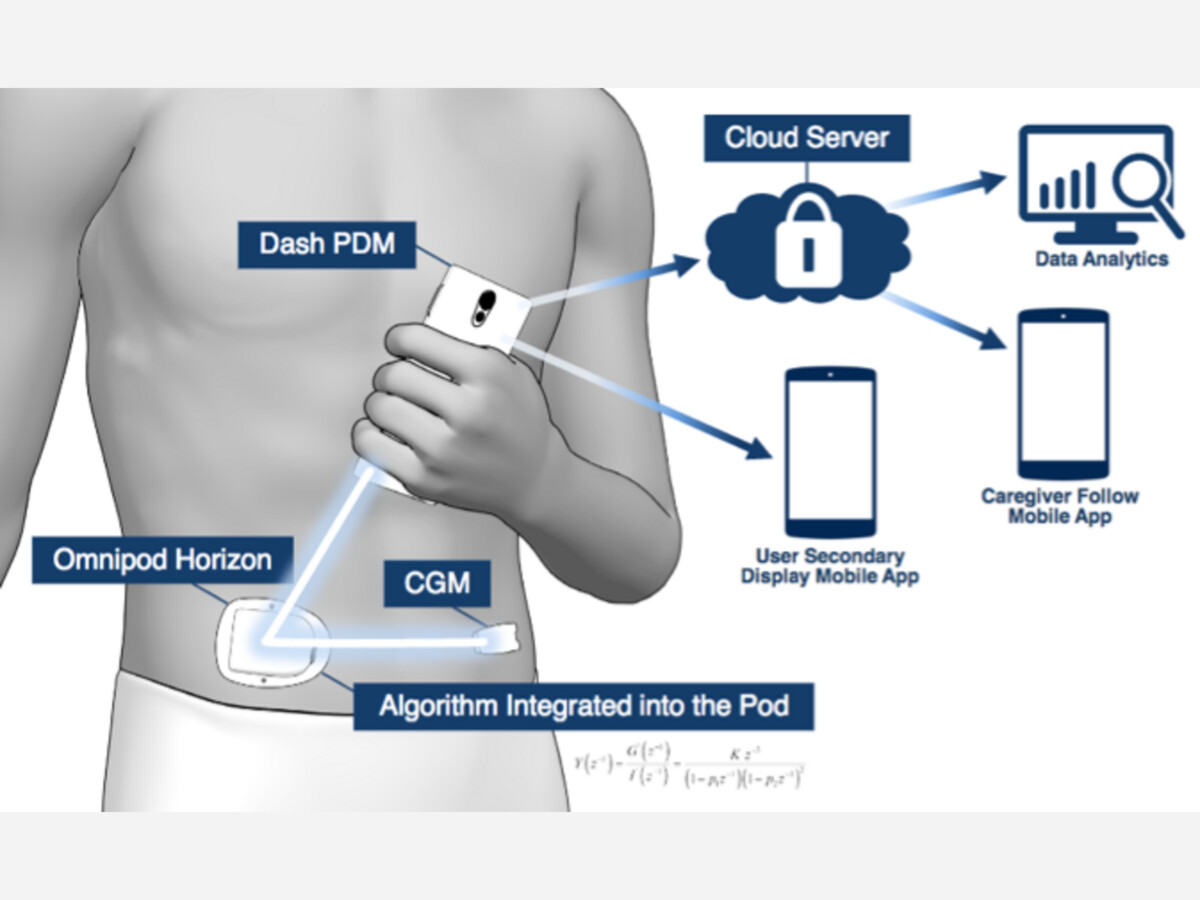


Insulet is recalling the Omnipod DASH Insulin Management System’s Personal Diabetes Manager (PDM) following reports of PDM battery problems, including battery swelling, fluid leakage from the battery, or intense overheating that may pose a fire hazard.
The U.S. Food and Drug Administration (FDA) has identified this as a Class I recall, the most serious type of recall. The use of these devices may cause serious injuries, serious health consequences, or death.
Users with questions can visit the Insulet website at www.omnipod.com/insulet-alerts or call 1-800-641-2049 to speak with the Insulet Customer Care team 24 hours a day, 7 days a week.
This report was shared by FDA MedWatch and has been updated.

Sunny, with a high of 66 and low of 53 degrees. Partly Cloudy during the morning, sunny during the afternoon, clear in the evening,